By Lee Helman, MD
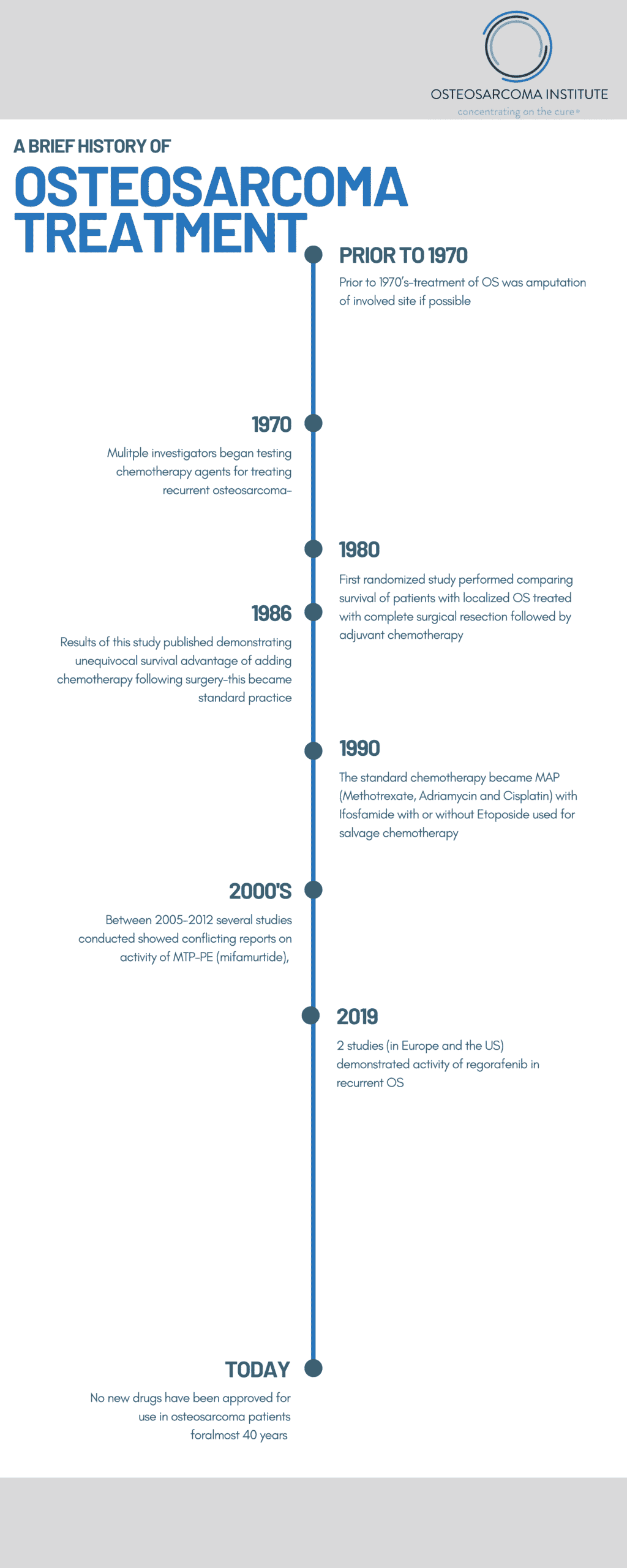
There has been a lack of improvement in outcomes for osteosarcoma patients over the last 40 years. Osteosarcoma is a very complex, recalcitrant disease that will be hard to cure. It will require the application of new concepts and technologies that are expensive to employ. As a physician who has dedicated my career to advancing treatments options in osteosarcoma, I often find inspiration in the experts that came before me to pave the way for further discoveries.
1970’s
Prior to the 1970’s, treatment for osteosarcoma began as an amputation of the effected limb upon presentation. With this approach, more than 80% of what was believed to be localized disease at that time died of metastatic disease usually to the lung within 2 years. In the early 1970’s that multiple investigators began testing chemotherapy agents for treating recurrent osteosarcoma. Dr. Norman Jaffee reported on the use of high dose methotrexate with citrovorum factor (leukovorum) along with vincristine after surgical resection in effort to reduce these high rates of recurrence in the lungs following surgical removal of the primary tumor. Dr. Jaffe reported a tripling of survival rates using this regimen, and several other investigators reported similar outcomes.
Surgical procedures also evolved to test whether amputations could be avoided with limb-sparing surgical resections and reconstruction of the effected limbs. This advancement was made possible by improved imaging techniques with CT scanning. Initially limb-sparing surgery limb-sparing surgery required a delay while waiting to receive a custom-fitted prosthetic bone implant. During this often several week delay, investigators began to give chemotherapy while awaiting arrival of the custom prosthesis. This led to reports suggesting benefits of giving pre-operative chemotherapy, including the ability to assess the effect of the chemotherapy directly on the tumor by examining the amount of necrosis seen in the resected specimen. Overtime, this became the standard approach, even though a randomized study comparing presurgical chemotherapy to immediate surgery followed by chemotherapy showed no statistical difference in outcome.
1980’s
Since none of the chemotherapy studies reporting improved outcomes carried out in the 1970s directly compared outcomes to patients received surgery alone, and there was much skepticism within the cancer field of these results, with many attributing these improved results to better surgery rather than the effect of chemotherapy. Given the controversy of the benefit of chemotherapy, a multi-institutional randomized study for patients with extremity, non-metastatic was begun in June 1982 comparing the effect of multi-agent chemotherapy including high-dose methotrexate with citrovorum factor rescue, Adriamycin, and cisplatin (MAP) (along with several other agents that have since found to be less active) versus observation following complete surgical resection. The study was reported in 1986 demonstrating that at 2 years an actuarial progression-free survival of 17% for patients on the control observation arm, versus 66% for the patients treated on the chemotherapy arm.
This method of treatment became standard practice in treating osteosarcoma patients.
1990’s
By 1990, with advancements of both imaging using CT scans and MRI scans, as well as uniform adoption of chemotherapy and further refinement, standard of care became pre-surgical chemotherapy, followed by mostly limb-sparing surgical resection, followed by additional post-surgical chemotherapy.
The standard chemotherapy became MAP (Methotrexate, Adriamycin and Cisplatin) with Ifosfamide with or without Etoposide used for salvage chemotherapy.
2000’s
The idea of stimulating an immune response to osteosarcoma dates back to the 1890s when a bone tumor surgeon, William Coley reported that injecting toxins derived from bacteria stimulated an inflammatory response leading in some cases to tumor shrinkage, including in bone tumors. This treatment not surprisingly led to toxicity including death and was eventually shut down. Nonetheless, interest in harnessing an immune response to osteosarcoma remained, and as knowledge of inflammation and immune responses improved, interest persisted. A synthetic derivative of bacillus Calmette–Guérin (BCG) know at MTP-PE was shown to have activity in dog studies of osteosarcoma. Studies published in 2005 and 2008 showed an improvement in 6-year survival from 70-78%, but there were problems with the data analysis from this study and in 2007 that ultimately prevented the FDA from approving MTP-PE, now known as mifamurtide. However, reviewing essential the same data, the European Medicines Agency approved mifamurtide for high-risk osteosarcoma in 2009, and this drug is currently still being studied in Europe and Mexico.
2010’s
During the late 1990s through 2010, multiple Phase 2 studies were conducted to identify new agents with activity in recurrent osteosarcoma. In each case, the results were negative, meaning there was no evidence of activity. Based on years of negative results, investigators have developed a new method to more rapidly look for hints of activity using smaller numbers of patients and trying to identify quickly new drugs that show improved progression free survival in patients with recurrent/relapsed OS. Using this newer approach to identify potentially active agents, several recent studies testing new drugs called tyrosine kinase inhibitors have shown some promise. At least 4 such type agents have been reported and two studies using a randomized approach testing regorafenib showed patients treated with this drug had improved early progression free survival. One study was conducted in Europe and one study was conducted in the U.S. Cabozantinib is a similar type of drug that seems to have some activity in recurrent OS.
2020’s
Based on the recent promising results testing several tyrosine kinase inhibitors in recurrent OS, we are encouraged that after years of negative results, we are at the beginning of a decade of promise in developing new approaches to improve the outcome of patients with OS, and OSI is confident that we can help accelerate this progress.